”Nowe organiczne i hybrydowe (organiczno-nieorganiczne) materiały i nanomateriały elektroaktywne o kontrolowanych właściwościach elektronowych, magnetycznych i optycznych”
„New organic and hybrid (organic-inorganic) electroactive materials and nanomaterials of tunable electronic, magnetic and optical properties”
Grant NCN OPUS 2019/33/B/ST5/00582
Kierownik grantu: prof. Adam Proń
Okres realizacji: 06.02.2020 – 05.10.2023
Przyznana kwota: 980 000 zł
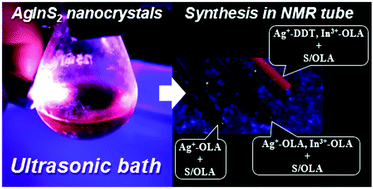
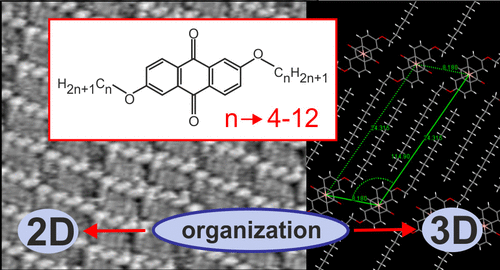
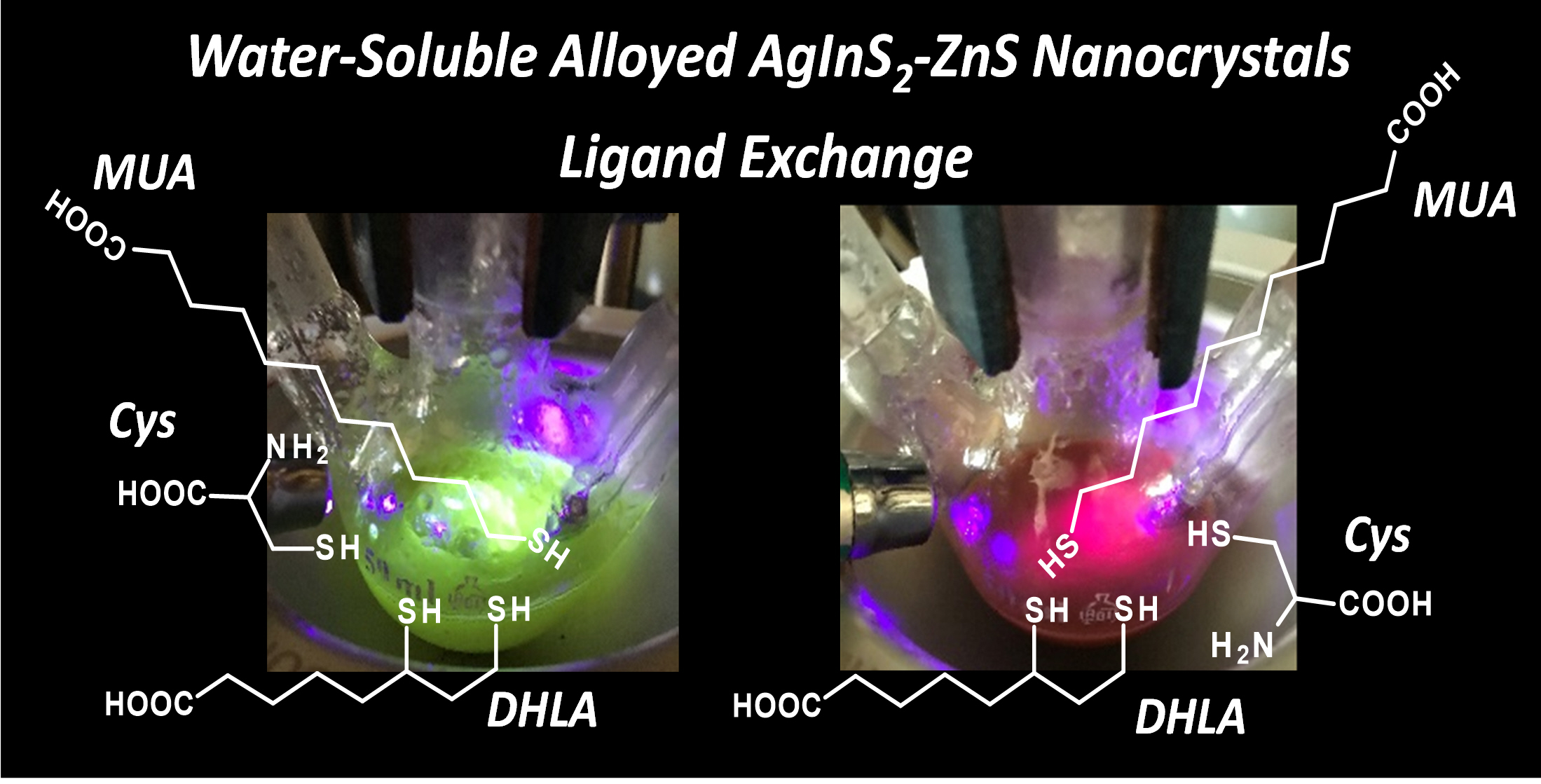
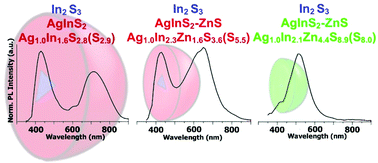
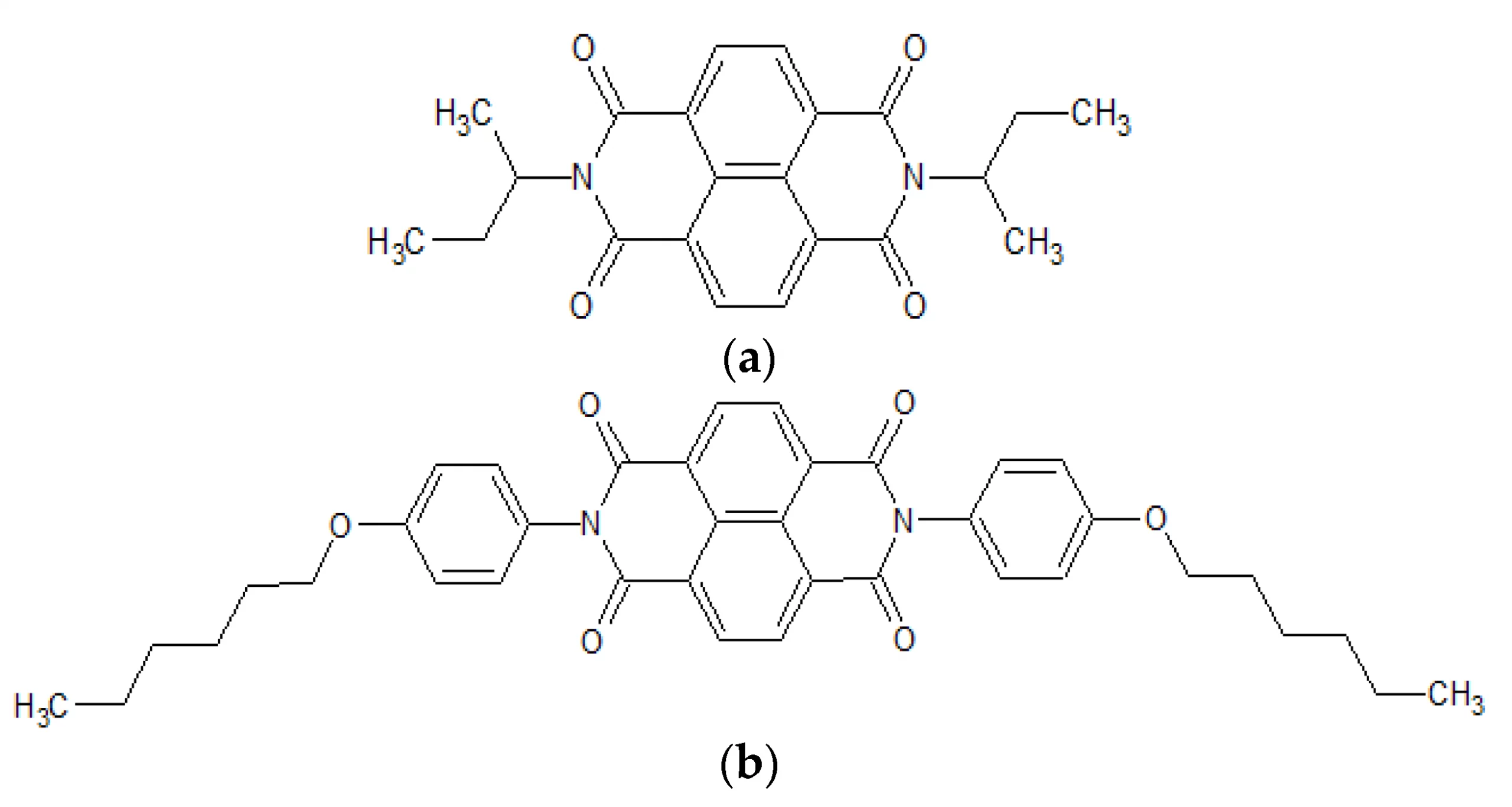
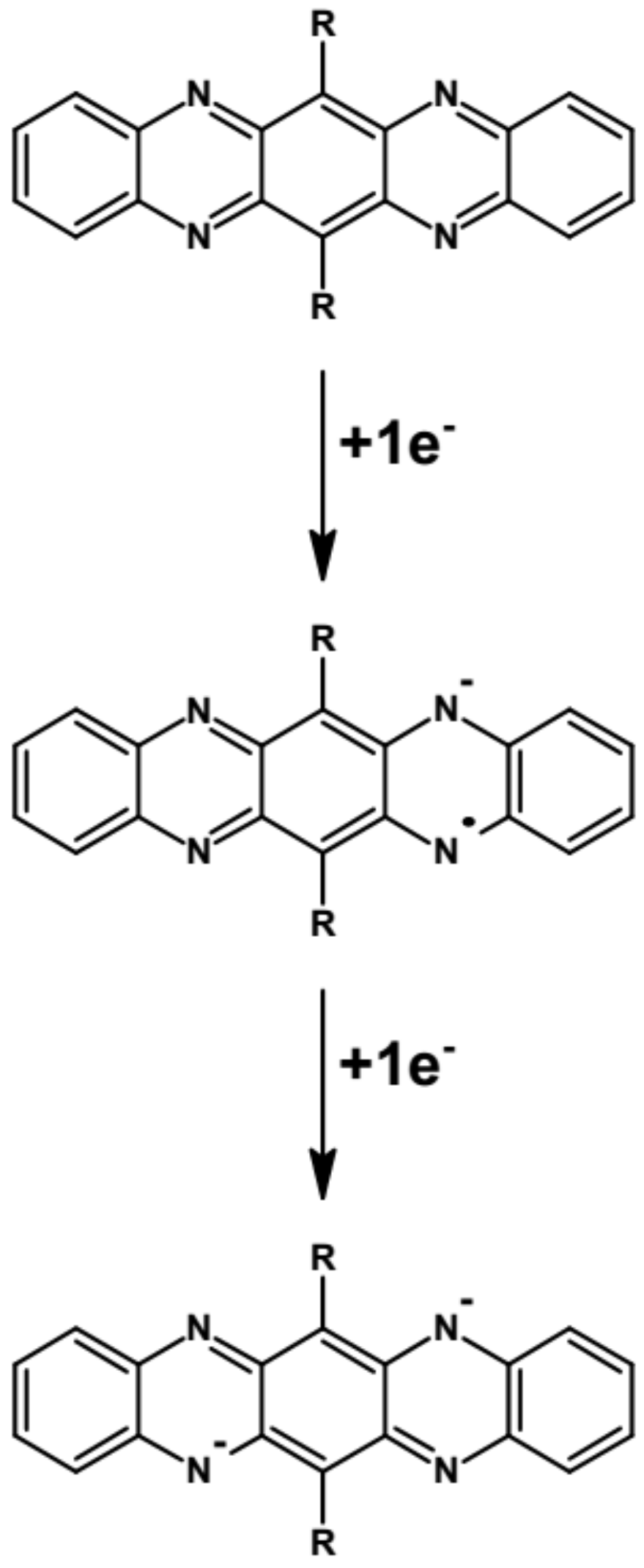
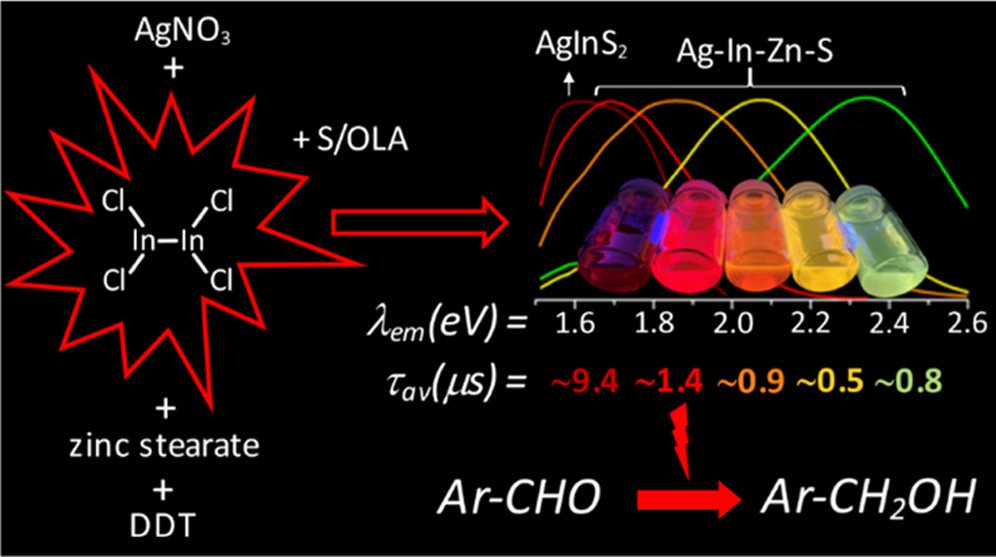
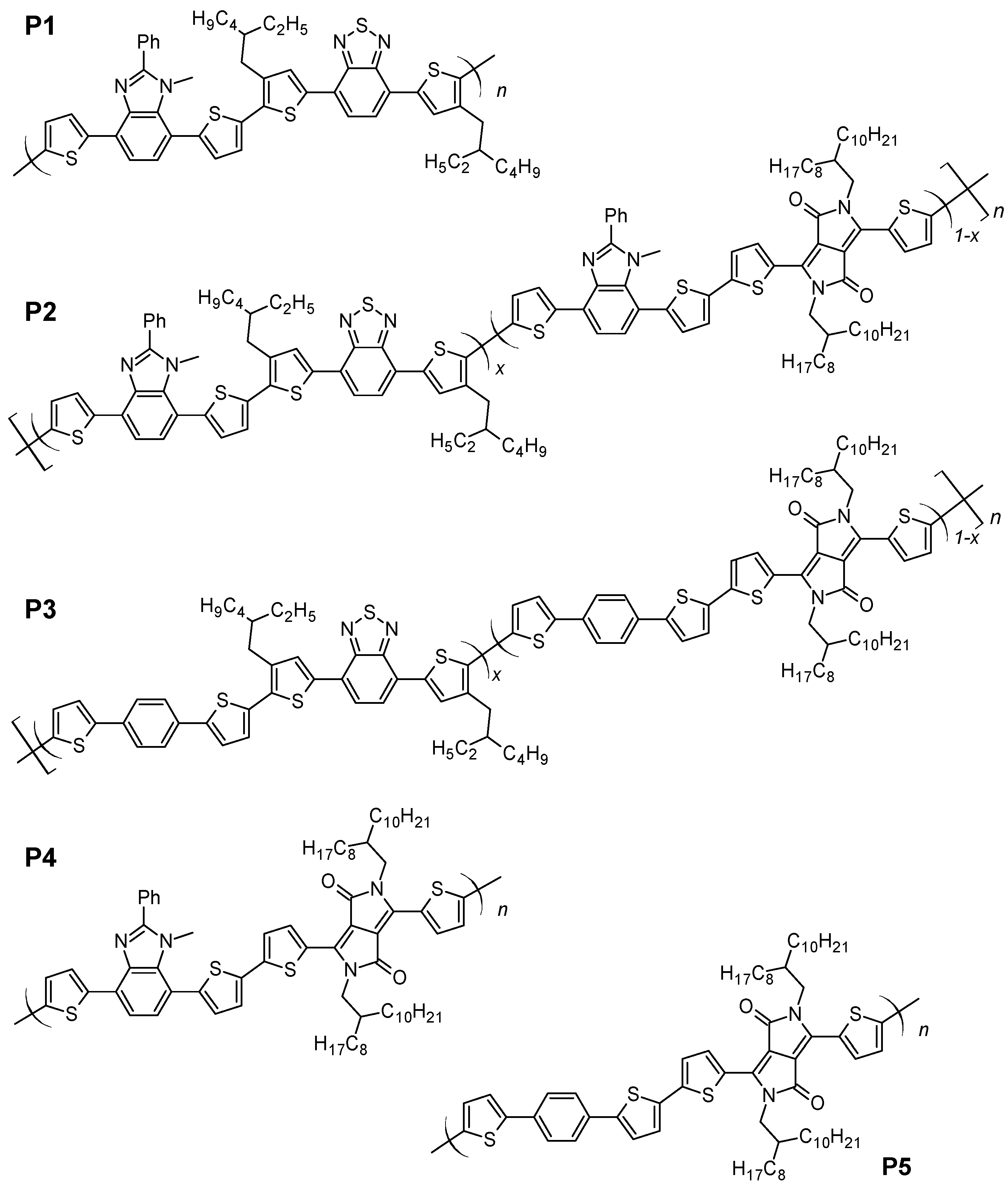
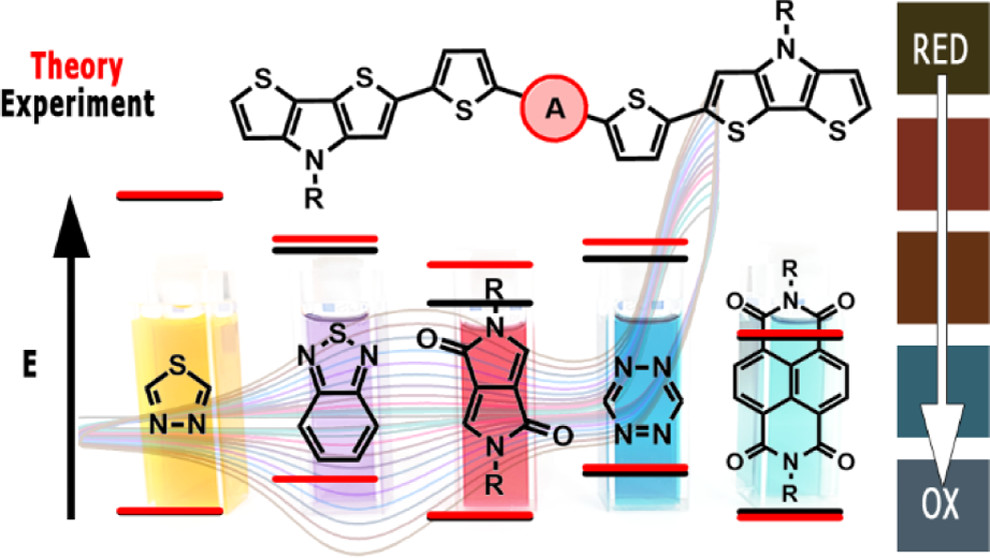
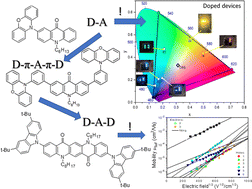
Abstract: The main goal of this project is to elaborate new low and high molecular mass organic and hybrid (organic/inorganic) semiconductor materials whose electronic, optical or magnetic properties could be tuned by appropriate functionalization leading to different molecule (macromolecule) topologies and appropriately adjusted intramolecular interactions. An important scientific novelty of the proposed project is to use various interactions such as electrostatic dipole moment, ferromagnetic spin-spin and charge transfer intramolecular interactions to improve electronic, magnetic or luminescent properties of the designed materials. This would also involve inorganic/organic hybrid materials in which electronic and optical properties will be tuned by interactions of inorganic nanocrystals with organic components occurring via especially designed conjugated linker ligands. New compounds will be synthesized in which following non-covalent interactions will be exploited for tuning their properties. Electrostatic interactions: As materials of potential interests in photovoltaics, new macromolecular compounds will be studied containing an intentionally incorporated moiety bearing a dipole moment. As dipole moment units benzoimidazole and acridone/quinacridone moieties will be used. Preliminary theoretical calculations showed that their dipole moments are equal to µ=3 D and 5 D, respectively and increase along the chain with increasing number of dipole bearing units. The dipole bearing units will be covalently incorporated into a conjugated polymer chain composed of donor segments (thiophene, dithienobenzo-dithiophene and/or dithienosilole) and acceptor units (benzothiadiazole and/or dipyrrolo-pyrrol-1,4-dione). Magnetic interactions: As high spin materials of potential use in organic spintronis, macromolecular compounds containing appropriately design and spin bearing units will be investigated. We propose a simplified strategy involving the incorporation of 2,7-naphthalenediyl as a spin coupling unit and 1,4-phenylenediamine and diaminophenylamine as spin bearing units. This strategy should ensure sufficient separation of adjacent charges, minimize the electrostatic repulsion and lead to the formation of co-extensive spin densities Charge and energy transfer interactions: New series of low molecular mass ADA and high molecular mass –(ADA)- and –(AD)- compounds will be synthesized. As D we will use quinolinoacridine derivatives which will be combined with A units varying in their acceptor strength. These compounds should exhibit ambipolarity, enhanced electrochemical cycling stability and tunable electronic, optical and redox properties. A derivative of these investigations will be devoted to the design of inorganic/organic hybrids consisting of inorganic semiconductor nanocrystals, conjugated surfacial ligands serving as transmitters and organic luminophores. The inorganic part will consist of highly luminescent ternary (Ag(Cu)-In-S (Se)) or quaternary (Ag(Cu)-In-Zn-S (Se)) nanocrystals free of toxic metals. To this inorganic core transmitting ligands will be attached to facilitate energy transfer from the nanocrystal to the target organic luminophores of nonlinear azaacene-type. All compounds designed with the help of DFT calculations will be synthesized using classical organic chemistry methods as well as palladium catalyzed C-C coupling (Suzuki, Stille and others) and C-N coupling (Hartwig-Buchwald) reactions. Special attention will be paid to regioregular polymers. The self-assembly capability and 2D organization of new semiconductors in monolayers will be investigated by scanning tunneling microscopy (STM). These studies will be completed by the determination of their 3D structure using X-ray diffraction techniques. Electrochemical and spectroelectrochemical (UV-vis-NIR, EPR, Raman) investigations will be carried out in addition to photoluminescence (stationary and time-resolved) studies. Photovoltaic properties will be determined in solar cells test devices. Spin interactions and multiplicity of new magnetic compounds will be determined by the use of pulsed-EPR spectroscopy, whereas macroscopic magnetic properties will be studied by the use of SQUID technique. The measurements of magnetization versus temperature will allow the exchange coupling constant determination.